Thermoflash LX260T Evolution Blanc
SARS-CoV-2 (Covid-19): Diagnosis by IgG/IgM Rapid Test
TO BE TESTED BY HOSPITALS OR INDIVIDUALS
For rapid detection of anti-SARS-CoV-2 IgG and IgM (2019-nCoV) in 10-15 minutes during Covid-19
COVID-19 is an infectious disease caused by the most recently discovered coronavirus, SARS-CoV-2 (2019-nCoV).
The rapid diagnostic test for SARS-CoV-2 allows qualitative detection of IgG and / or IgM in serum, whole blood or human plasma in approximately 10-15 minutes.
The rapid tests are based on the principle of lateral flow immunoassay chromatography and are available in cassette form. The test is based on the separation of the components of a mixture through a medium using capillary force and the specific and rapid binding of an antibody to its antigen.
IgM and IgG are immunoglobulins produced by the immune system to protect against SARS-CoV-2. Anti-SARS-CoV-2 IgM and IgG can therefore be detected in samples from affected patients.
We offer several types of kits to detect IgG or IgM or both at the same time. We also offer kits containing only cassettes and other complete kits for sampling and testing. All these kits are intended for in vitro diagnostic use and can only be used by healthcare professionals.
PRACTICE FOR MASS SCREENING
COVID-19 IgM / IgG rapid test
This test detects both early and late markers, IgM / IgG antibodies in human finger prick or venous blood samples.
It can be used for the rapid detection of carriers of the virus who are symptomatic or asymptomatic. Recent studies suggest that a high percentage of patients have no clinical symptoms of the virus, so patient screening is of vital importance. This test can be easily administered to anyone. The test can also be deployed effectively in companies, schools, airports, seaports and stations, etc., which allows it to become a key force in the fight against this global threat.
What is the principle of rapid tests for SARS-CoV-2
The test detects the presence of antibodies produced by the patient against SARS-CoV-2, the virus that causes COVID-19 disease. The test can detect two types of antibody isotypes: IgG and IgM.
There are several types of tests, but the most common is to attach anti-human IgG and IgM antibodies to the surface of the cassette and to couple a virus antigen with colloidal gold particles. If the patient sample contains anti-SARS-CoV-2 antibodies, these antibodies will bind to the antigen present in the cassette conjugation buffer and the complex formed will migrate to bound anti-human IgG and / or IgM to the membrane. A colored band will then appear (see photos for possible results).
Features and benefits
The Rapid IgM-IgG combined test for COVID-19 is used to qualitatively detect the IgG and IgM antibodies of the new coronavirus in human serum, plasma or whole blood in vitro.
Specific
Works with whole blood, serum and plasma
IgM and IgG antibody tests
Sensitivity: 98.7%
Specificity: 91.3%
10-15 minutes per test
Intuitive visual interpretation
No special equipment required
Easy test administration
How it works
4 simple steps
1. Take a blood / serum / plasma sample.
2. Add a blood / serum / plasma sample for proper sampling.
3. Place 2 to 3 drops of buffer in the sample well.
4. Read the results after 10 minutes and not more than 15 minutes.
Technology
The new rapid test provides an accurate diagnosis of COVID-19 infection in 15 minutes.
It is widely believed that IgM provides the first line of defense during viral infections, followed by the generation of high affinity adaptive IgG responses for long-term immunity and immunological memory.
Therefore, the COVID-19 IgM and IgG antibody test is an effective method for the rapid diagnosis of COVID-19 infection. In addition, detection of COVID-19 IgM antibodies tends to indicate recent exposure to COVID-19, while detection of COVID-19 IgG antibodies indicates a later stage of infection. Thus, this combined antibody test could also provide information on the stage of infection.
It is therefore a quick service to help in the diagnosis of coronavirus infection. The test detects both early and late markers, IgM / IgG antibodies in human finger (capillary) or venous, serum and plasma samples. Serological tests are capable of detecting COVID-19 antibodies for an extended period of time after resolution of the disease, which helps identify a previous infection. Knowledge of a previous infection is epidemiologically important and represents a significant unmet need in the management of the COVID-19 pandemic.
distribution of this product for use in laboratories certified to perform high complexity tests
What are the advantages of rapid tests:
Rapid screening in 10 to 15 minutes.
High detection efficiency: simultaneous monitoring of IgM and IgG.
Detection without any test equipment.
Easy to use and compatible with serum / whole blood / plasma.
Storage at room temperature.
What is the principle of rapid tests for SARS-CoV-2
The test detects the presence of antibodies produced by the patient against SARS-CoV-2, the virus that causes COVID-19 disease. The test can detect two types of antibody isotypes: IgG and IgM.
There are several types of tests, but the most common is to attach anti-human IgG and IgM antibodies to the surface of the cassette and to couple a virus antigen with colloidal gold particles. If the patient sample contains anti-SARS-CoV-2 antibodies, these antibodies will bind to the antigen present in the cassette conjugation buffer and the complex formed will migrate to bound anti-human IgG and / or IgM to the membrane. A colored band will then appear (see below for possible results).
The level of IgM antibodies begins to increase after 1 week after the initial infection, while the IgG appears later than the IgM (generally 14 days after infection) and can last 6 months or even several years, which which means that IgG serves as an indicator of a previous infection. Suspected patients infected with SARS-CoV-2 can be quickly identified by simultaneous monitoring of IgM and IgG. During the epidemic period of 2003-SARS and 2016-Zika, detection of IgM / IgG antibodies was used as one of the recommended diagnostic methods.
A sample can be positive if IgM, IgG or IgM and IgG antibodies are present.
There are different cassettes for quick tests. In general, for qualitative detection of IgG and IgM at the same time, there are 3 different lines: one for IgG, one for IgM and one for control.
To be validated, this test must have a positive line for the control (C)
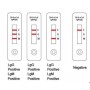
Results
Interpretation
IgM + / IgG +
Recent SARS-CoV-2 infection
IgM + / IgG-
Recent SARS-CoV-2 infection
IgM- / IgG +
Previous SARS-CoV-2 infection
IgM- / IgG-
No infection or not enough detectable antibodies at the start of the infection
Medical devices for in vitro diagnostics. Read the instructions carefully.
All these kits are intended for in vitro diagnostic use and can only be used by healthcare professionals.
FAQ
Is the COVID-19 IgM-IgG rapid test available in the United States?
On May 4, 2020, the FDA updated its policy for diagnostic tests for 2019 coronavirus disease during the public health emergency. The document includes guidance for commercial manufacturers such as BioMedomics for serological tests that identify antibodies (eg, IgM, IgG) against sARS-CoV-2 from clinical samples. The policy authorizes tests in laboratories certified to perform high complexity tests and at the point of service when they are covered by the laboratory's CLIA certificate for high complexity tests. The policy does not apply to home testing.
Labs and healthcare providers should include this information in their patient test report as specified in the FDA guidelines:
This test has not been reviewed by the FDA
Negative results do not rule out SARS-CoV-2 infection. If an acute infection is suspected, a direct test for SARS-CoV-2 is necessary.
Antibody test results should not be used to diagnose or exclude SARS-CoV-2.
Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as HKU1, NL63, OC43 or 229E coronavirus.
What is the COVID-19 IgM-IgG rapid test?
Rapid IgM-IgG Combined Antibody Test for COVID-19 is a lateral flow immunoassay used to qualitatively detect IgG and IgM antibodies to the new coronavirus in human serum, plasma or whole blood in vitro.
This test should not be used with a heat-inactivated or other inactivated human sample (blood, serum, plasma). Fresh samples should be taken and tested immediately.
How does the COVID-19 rapid test work?
The test strip contains a new recombinant coronavirus antigen labeled with colloidal gold and a colloidal gold marker for quality control antibodies, two lines of detection (lines G and M) and a line of quality control ( C) fixed on a nitrocellulose membrane. M is fixed with a monoclonal anti-human IgM antibody to detect the new IgM antibody against the coronavirus. G is fixed with a monoclonal anti-human IgG antibody to detect the new anti-coronavirus IgG antibody. The quality control antibody is attached to line C.
How fast can the COVID-19 rapid test work?
Results are valid 10 minutes after the sample and buffer have been combined in the cassette sample well.
How accurate is the COVID-19 rapid test?
The COVID-19 IgM-IgG rapid test is intended to test IgM and IgG separately. The test was validated against a panel of previously frozen samples comprising thirty (30) serum samples positive for SARS-CoV-2 and seventy (70) serum and plasma samples negative for antibodies. Each of the 30 antibody positive samples was confirmed by a nucleic acid amplification test (NAAT) and the antibodies IgM and IgG were confirmed to be present in the 30 samples. The presence of antibodies in the samples was confirmed by several orthogonal methods before the test with the COVID-19 IgM-IgG rapid test. The presence of IgM and IgG antibodies was specifically confirmed by one or more comparison methods. Antibody positive samples were selected for different antibody titers. All negative antibody samples were collected before 2020 and include 70 samples selected regardless of clinical status ("negative"). The test was performed by an operator using a batch of the COVID-19 IgM-IgG rapid test. Confidence intervals for sensitivity and specificity were calculated using a scoring method described in CLSI EP12-A2 (2008). Antibody positive samples were selected for different antibody titers. All negative antibody samples were collected before 2020 and include 70 samples selected regardless of clinical status ("negative"). The test was performed by an operator using a batch of the COVID-19 IgM-IgG rapid test. Confidence intervals for sensitivity and specificity were calculated using a scoring method described in CLSI EP12-A2 (2008). Antibody positive samples were selected for different antibody titers. All negative antibody samples were collected before 2020 and include 70 samples selected regardless of clinical status ("negative"). The test was performed by an operator using a batch of the COVID-19 IgM-IgG rapid test. Confidence intervals for sensitivity and specificity were calculated using a scoring method described in CLSI EP12-A2 (2008).
The COVID-19 IgM-IgG rapid test showed a combined sensitivity of 96.7% and a combined specificity of 97.1%.
What do the results tell me?
A total of three detection lines is possible, the control line (C) appearing when the sample has passed through the cassette.
1 | Negative result: if only the quality control line (C) appears and the detection lines G and M are not visible, no new coronavirus antibodies have been detected and the result is negative.
2 | Positive result, M only: if the quality control line (C) and the detection line M appear, then the new IgM antibody against the coronavirus has been detected and the result is positive for the IgM antibody.
3 | Positive result, G only: if the quality control line (C) and the detection line G appear, then the new IgG antibody against the coronavirus has been detected and the result is positive for the IgG antibody.
4 | Positive result, G and M: if the quality control line (C) and the two detection lines G and M appear, then the new coronavirus antibodies IgG and IgM have been detected and the result is positive for the IgG antibodies and IgM.
What are the alternatives?
The COVID-19 IgM / IgG rapid test can be used to screen patients suspected of having been affected by the new coronavirus. However, test results should not be the only basis for diagnosis. The results should be used in combination with clinical observations and other testing methods such as the nucleic acid PCR test.
Returns Policy
You may return most new, unopened items within 14 days of delivery for a full refund. We'll also pay the return shipping costs if the return is a result of our error (you received an incorrect or defective item, etc.).
You should expect to receive your refund within four weeks of giving your package to the return shipper, however, in many cases you will receive a refund more quickly. This time period includes the transit time for us to receive your return from the shipper (5 to 10 business days), the time it takes us to process your return once we receive it (3 to 5 business days), and the time it takes your bank to process our refund request (5 to 10 business days).
If you need to return an item, simply login to your account, view the order using the 'Complete Orders' link under the My Account menu and click the Return Item(s) button. We'll notify you via e-mail of your refund once we've received and processed the returned item.
Shipping
We can ship to virtually any address in the world. Note that there are restrictions on some products, and some products cannot be shipped to international destinations.
When you place an order, we will estimate shipping and delivery dates for you based on the availability of your items and the shipping options you choose. Depending on the shipping provider you choose, shipping date estimates may appear on the shipping quotes page.
Please also note that the shipping rates for many items we sell are weight-based. The weight of any such item can be found on its detail page. To reflect the policies of the shipping companies we use, all weights will be rounded up to the next full pound.